Professor Jane Visvader and Professor Geoff Lindeman lead a team that discovers breast stem cells, showing a whole and fully functional mammary gland can grow from a single breast stem cell.
The discovery changes the course of our understanding of the origins of breast cancer.
What makes a breast?
The female breast has been the object of considerable interest over the millennia. Yet for all its close examination in the annals of art and literature, as recently as 1997 remarkably little was known about the science of what makes a breast.
So an opportunity that year to come to Melbourne to begin a deep investigation into breast biology and development, and what it might teach about how to tackle cancer, was too good for Professor Jane Visvader and her collaborator (and husband) oncologist Professor Geoff Lindeman to resist.
The invitation to the pair was to establish a breast cancer laboratory at the Walter and Eliza Hall Institute. At the time they were in Boston in roles attached to the Harvard Medical School. Visvader was working in the company of some stellar scientists investigating transcription factors – proteins that control which genes are turned on or off – in the haemopoietic (blood production) system. But in such a crowded research landscape how, she wondered, might she make her mark?
Breast cancer conundrum
“When this opportunity came along I did some reading and was really struck by the paucity of knowledge on normal mammary gland development and the molecules that guide it.” Without an understanding of normal processes, how could science begin to unravel what goes awry and causes cancer? In such untraveled territory there was the prospect of making a significant impact – or failing spectacularly.
Adelaide born-and-educated, Visvader had worked at the Walter and Eliza Hall Institute early in her career. It was there that she met Lindeman when they were serving their scientific apprenticeships under the tutelage of another romantic and scientific partnership, Professor Jerry Adams and Professor Suzanne Cory.
By 1997 Cory was the new director of the institute, and head-hunted Visvader and Lindeman to come back to set up the laboratory under the auspices of the Victorian Breast Cancer Research Consortium, a visionary initiative of the then Victorian Government.
“We were very fortunate that they had faith in us,” says Visvader. “Though we were both involved in different aspects of cancer research, neither of us knew anything about mammary gland development. It was a bit daunting, coming into a lab with no molecular or cellular tools. But we gradually built them up, with help from other labs in Australia and around the world.”
But soon it became clear why breast development and its molecular regulation had been so long neglected. It was formidably complex work, much more so than working with the blood compartment. “It took us years to successfully disaggregate the tissue and end up with viable cells.”
A breakthrough came by exploiting the institute’s renowned animal facilities and “capacity for doing meticulous cells sorting from the blood, bone marrow and other organs. We thought we’d attempt to apply this to the mammary gland. It took us almost five years of intensive work.”
Breast stem cells discovered
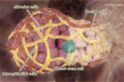
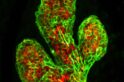
The result was a watershed 2006 Nature paper isolating and identifying the mouse mammary stem cell. It documented the team’s success in growing a functional, milk-producing mammary gland in a mouse from a single stem cell obtained from the mammary gland of another mouse.
The achievement cracked open exploration of the origins of different types of breast cancer and ways in which they might be controlled.
“We could start to ask important questions. What is the nature of the hierarchy of cells within this tissue? Which cells are predisposed to becoming cancerous? How do they relate to the different subtypes of breast cancer? The identification of breast stem cells was our eureka moment, and without it, we would not have been in any position to ask the next set of questions.”
They discovered that their mouse models were very potent tools for their research, because of the close similarities between the cellular constituents in mice and in women. As well as explaining the deep molecular mechanics of what’s at play, these models provide the springboard into developing therapeutic solutions. By growing human tumor cells in mice they could test the efficacy of potential drugs and pave the way toward human clinical trials.
The team’s leadership provides an advantage in straddling the bench-to-bedside research gap. “Because I’m a pure scientist, and Geoff is a clinician-scientist, we collaborate very closely, and can span molecular and cellular biology, as well as animal biology and use that to drive translational research.
“You’ve got to let fundamental science dictate the clinically relevant questions as good practice requires a very firm base.”
Identifying the culprits
By identifying the stem and their ‘daughter’ cells that reside in normal breast tissue, and understanding how they go off track to initiate cancers, the team can work toward improving diagnostic and prognostic markers. Ultimately the goal is to develop drugs with the power to prevent that cascade beginning. This has potential to provide protection for families with high genetic risk of breast cancer.
Another milestone in the team’s progress was in identifying the culprit in BRCA1 mutation carriers – who have a much higher risk of breast and ovarian cancers – as the daughter cell and not the stem cell.
“It became clear that the daughter cell is the one that is predisposed to generating the basal cancers, a very aggressive type of breast cancer, not only in the BRCA1 mutation carriers but also in the 15 to 20 per cent of women who develop sporadic cases of basal breast cancer.”
This opens the prospect of finding ways to switch the errant daughter off. “This is why understanding the cellular origins of a particular type of cancer is an important question,” says Visvader.
New breast cancer treatments
Her team has identified a possible mechanism for intervening with expansion of these daughter cells using a drug currently used to treat osteoporosis, and a clinical trial is being initiated by Lindeman, enlisting women who don’t have breast cancer but do have the BRCA1 mutation.
“It holds great promise, but we will not know the outcome for some time.”
Other research avenues include building on the institute’s rich history with discovering and exploiting the Bcl-2 family of proteins that regulate cell death and survival and using combination therapies to ensure the cells that initiate cancers are wiped out and don’t return.
This builds on substantial gains in breast cancer treatments in recent times, drugs like Tamoxifen and aromatase inhibitors (which stop the production of oestrogen in postmenopausal women) “have completely revolutionised breast cancer therapy for 70 per cent of women. For many of those the prognosis is now extremely good.”
While there are still classes of cancer that are frustratingly complex and devious in their mechanisms “there is a lot of hope”, says Visvader. “The heterogeneous nature of breast cancer makes it very tricky, but the gains in even the past decade show us it is very worthwhile.”