Dr Marc Pellegrini, Dr Greg Ebert and colleagues develop a new treatment to eliminate hepatitis B in collaboration with TetraLogic Pharmaceuticals, a biotech company based in Malvern, Pennsylvania, US.
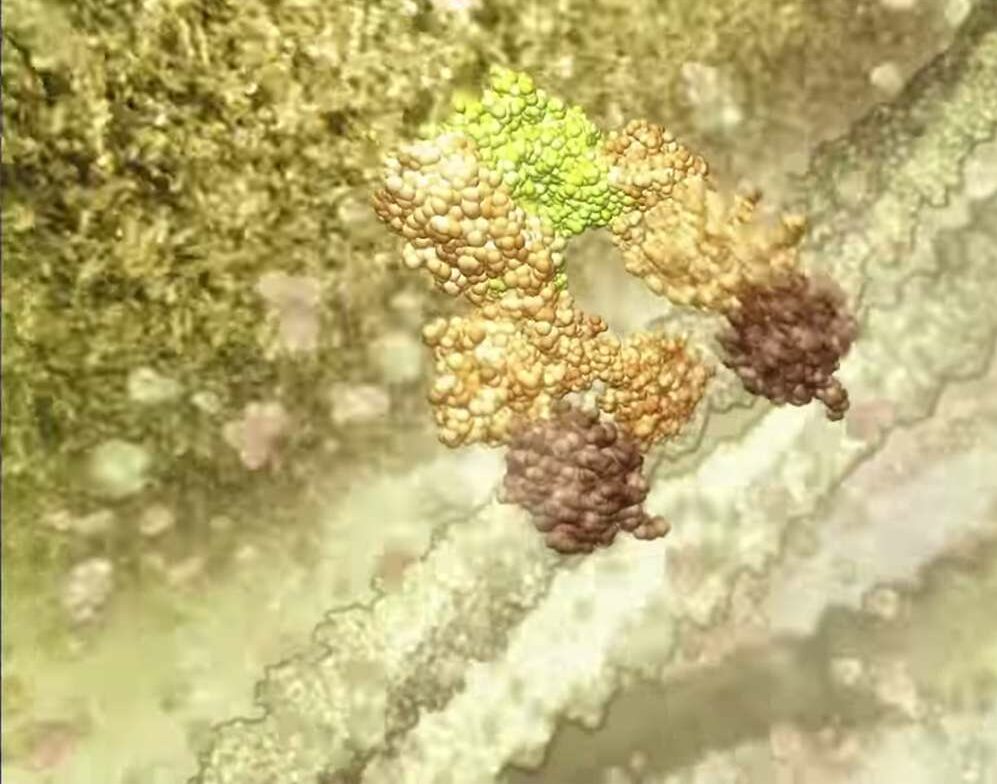
The treatment uses TetraLogic Pharmaceutical’s anti-cancer drug birinapant, which triggers the breakdown of proteins that prevent virus infected cells from self-destructing. Pellegrini says these proteins, known as ‘inhibitors of apoptosis proteins’ (IAPs), can be targeted to allow infected cells to die.
Stalled but not vanquished
As a medical student in the early 1990s, Pellegrini watched his fraternity grapple with emerging infections, especially HIV. The new combination of antiretroviral drugs was becoming available, but the optimism was soon tempered by the realisation that the virus could be stalled, but not vanquished.
“As we were going through all this it became clear that we couldn’t cure people at all,” recalls Pellegrini, senior researcher and clinician at the Walter and Eliza Hall Institute. “It made me interested in finding cures for all these infections for which we have medicines to control, but not cure the disease— infections that still cause morbidity.”
After specialising in infectious diseases, completing his training at Melbourne’s St Vincent’s and Fairfield hospitals, he arrived at the Walter and Eliza Hall Institute to undertake his PhD and tackle the problem from an innovative perspective.
Boost immunity to fight infections
Rather than attack the virus, Pellegrini seeks to boost the body’s immune response. The common denominator in “overwhelming infections” such as HIV, hepatitis B, tuberculosis, the herpes virus and dengue fever is that the virus “commandeers all the machinery of a cell,” wiping out the sophisticated defence mechanism that causes an infected cell to sacrifice itself for the greater good.
“Generally when a cell is invaded by a pathogen there’s a safety switch that recognises that abnormality,” Pellegrini explains. “And this switch causes the cell to commit suicide altruistically to protect the whole body. The reason we don’t get cancer more regularly is that, for the most part, this innate program works.
“HIV, for instance, takes up residence in a T cell (a type of white blood cell) and takes over the machinery in a way that mimics the cancer cell. The cell is highly abnormal— but in HIV the abnormality isn’t recognised so the cell survives.”
Cell life and death
Pellegrini embarked on his PhD at a time when “huge groups were being set up looking at the processes that dictate whether a cell responds to the external environment – how cells survive and how they die”. He joined one such group, the Molecular Genetics of Cancer division, working under Professors Andreas Strasser, Jerry Adams and Suzanne Cory.
After his PhD, Pellegrini’s postdoctoral work took him to the Campbell Family Institute for Breast Cancer Research in Toronto where he further explored the question of how to ‘convince’ abnormal cells to commit suicide. On his return to the Walter and Eliza Hall Institute in 2009, he set up a new lab with a new team to study human infections according to this new paradigm.
Convincing abnormal cells to self-destruct
Rather than finding agents or antibiotics to target bugs only to see those bugs “adapt reasonably successfully” and become resistant to the drugs, the aim was to convince abnormal cells to self-destruct so the bugs have no comeback. The task is to ‘repurpose’ cancer drugs to combat infectious diseases.
Seven years on Pellegrini can point to several new discoveries. Most significantly, a drug therapy that successfully targeted the hepatitis B virus in preclinical trials progressed to human phase 1 trials in October 2014
“To get from a very basic problem or question to a clinical trial in four years was really exciting and unusual,” Pellegrini reflects. He attributes his success partly to the institute’s culture of collaboration across research divisions and its established contacts with pharmaceutical companies.
For now, the biggest challenge – aside from convincing pharmaceutical companies to fill the gaps in medical research funding – is getting clinicians to support the trials and progress them further.
“Traditionally there’s been a divide in Australia between doctors and scientists. They don’t speak the same language. But I’m both – a researcher and a clinician. I speak both languages and I think that helps.”