Dr Anne Verhagen, Professor David Vaux and colleagues discover the first protein in mammals that triggers cell death by binding to and antagonising IAP proteins.
Removing the negative
To make space for the millions of cells your body makes every second, millions of cells must also die. Cells have a finite lifespan and when they reach the end of their life – or become infected or inefficient – they trigger a cell death mechanism called apoptosis.
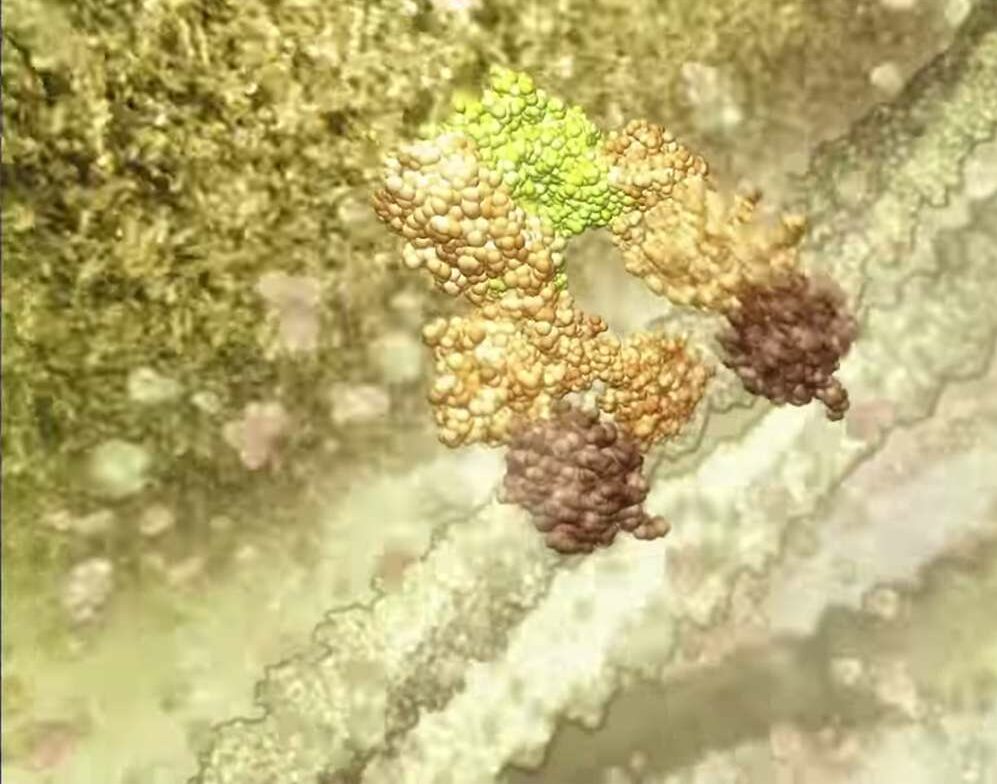
Building on the institute’s discoveries of Bcl-2 and inhibitor of apoptosis proteins (IAPs) that prevent programmed cell death, Verhagen, Vaux and colleagues discover a protein that binds to IAPs. This protein, called Diablo (also known as Smac), enables apoptosis to occur by antagonising the IAPs that block apoptosis themselves. By removing the negative action of IAPs, cell death can proceed.
Platform for new anticancer drugs
This discovery is the catalyst for a research effort to create new anticancer drugs called smac-mimetics.
These drugs are designed to perform the same action as Diablo and associated proteins, triggered cell death. Drugs based on this discovery are in clinical trial for use against cancers such as leukaemia and lymphoma, and infectious diseases including hepatitis B.
Signing the death warrant
If cells become damaged and need to invoke their suicide program, the IAPs must first be neutralised. The mechanism was unknown until the first opponent of IAPs was recently discovered by researchers in the Molecular Genetics of Cancer division and the Ludwig/Walter and Eliza Hall Institute Protein Structure Laboratory.
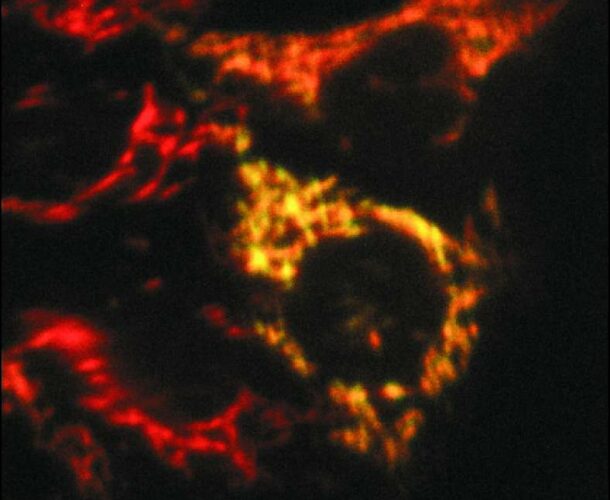
Dubbed Diablo, this protein lurks inside mitochondria, the energy-producing factories within our cells, where it can be stained with fluorescent antibodies. When the mitochondria receive notice of the cell’s death warrant, Diablo emerges and binds to IAPs, whereupon they release their death cargo of caspases.
A seismic impact on cell biology
One weekend in late 1999, not long before their second baby was due, Dr Anne Verhagen and her husband, Dr Peter Lock, spent an anxious weekend trying to think of a name. Not for the baby – that could wait a little longer. Hopefully.
The challenge on this weekend was to come up with a name for the human protein Verhagen had discovered after years of painstaking investigation, one destined to have a seismic impact in cell biology and with powerful potential in cancer treatment. The rush was on to publish the news – and the name – in the prestigious journal Cell.
With another team in the US about to publish related findings, it was down to the wire to get the work of Verhagen and her Walter and Eliza Hall Institute colleagues acknowledged in a watershed joint publication, or be left in the dust.
Verhagen pulled out all stops to get the paper delivered before the baby. Such determination seems true to type. Raised in Victoria’s La Trobe Valley in the 1980s, she had defied the modest expectations of many students of that place and time to secure a science PhD, a coveted fellowship to Germany, and a place in Professor David Vaux’s famously bold cancer research laboratory.
Learning to throw the safety switch
The Vaux team was investigating how cells kill themselves – or not. It focused on the molecules that control the destiny of cells, because having too many or too few can cascade into diseases including cancers. The race was on to learn how to safely throw the switch to activate or inhibit cell death, and potentially stop malignancies in their tracks.
Some years earlier scientists had described these mechanisms in flies, and identified some proteins that could bring on cell death. “They all had cool names – Grim, Reaper and Hid,” explains Verhagen. “They would antagonize the inhibitor of apoptosis proteins (IAPs), and you would get death.”
Might something similar exist in mammalian systems? This is what she spent three years – pausing for baby number one – trying to find out. Applying some biochemical tricks learnt overseas, she used the IAP protein as a bait to fish for other potentially important regulatory proteins. At a time when databases for gene and RNA sequences were incomplete, the process required manually piecing together scraps of protein and genetic sequence information – think of it as a fiendishly difficult, microscopic jigsaw puzzle.
Yet she’d nailed it – with the help of colleagues in the Vaux laboratory, postdocs Paul Ekert and John Silke, research assistant Miha Pakusch and experts in the Walter and Eliza Hall Institute-Ludwig Institute Joint Protein Structure Laboratory headed by Professor Richard Simpson.
First comes the protein then comes the baby
After a marathon push to pull all the work together, just one thing remained before it could be sent off for peer review. So far they had been referring to their discovery as “spot 4”, but that would never do.
It was Peter (Verhagen’s husband – also a scientist) who coined the term Diablo, which stands for Direct IAP binding protein with low pl – stretching it a little, but a great name, Verhagen recalls.
Vaux heartily approved, typed it into the manuscript, and sent it off.
But the drama wasn’t over yet. Then came the baby, the much less ominously named Charlie. When Vaux dropped by with a gift for the newborn, he also brought news that the Cell reviewers had some more urgent questions. He was recruited to jiggle three-week-old Charlie in an office next to the laboratory while Verhagen resolved the outstanding issues with further experiments.
“Dave Vaux would change Charlie’s nappy. I’d come out and feed him. It was pretty intense. Then I wrote it up at home, baby on boob. This time it was accepted.” The paper was a blockbuster, and the insights it yielded are still playing out.
“Drug trials are underway with molecules that mimic the function of Diablo in cancer treatments,” Verhagen says. “It was always exciting as a potential cancer drug.”
Women in science forging ahead
While much of the thrill of basic research for Verhagen was in crafting her technique, a unique tool with which to excavate key morsels of critical cellular information, “as somebody going into biology and research, you always have ideas about curing disease. And that’s definitely why I went into it.”
Her triumph, and the energy and support it required to bring it off, provides a graphic illustration of the pressures women researchers wanting families inevitably encounter. After years acquiring qualifications and skills their careers hit peak hour at the same time as their prime baby-making years. The issue is further complicated by funding paradigms that, at least until lately, have been unforgiving of family demands.
The consequence of these dynamics is plain in the vanishing of women from senior positions at the institute, despite females accounting for half the post-docs and PhDs entering the institute. It’s a priority concern for director Professor Doug Hilton.
An everlasting impact
Verhagen credits Diablo to the good fortune of working with a collaborative, supportive team, and having a boss who had a young family himself. “It may not have happened in another institution. Maybe not in another lab. It was that lab, in that moment in time – everyone wanted this paper.”
She continued at the institute in for another six years (and another baby), accumulating more than 30 papers in cell biology, cellular biochemistry and immunology, then stepping away to spend time with her children. “I felt I would regret not having spent this extra time with them, more than I would regret an impact on my career. But I knew it would impact, and of course it did.”
Today Verhagen is head of CSL’s cellular biochemistry group, which allows her to take a dynamic role in translating research into the treatment of various diseases. It’s rewarding frontline work, engaging collaboratively with a range of institutions, including the Walter and Eliza Hall Institute.