Scientists discover a gene that holds the key to cancer cell death, causing a revolution in what we know about cancer development.
It is the spark that leads to the discovery and development of anti-cancer drugs now in clinical trials for treating people with leukaemia, and with promise for many more cancer patients.
A cancer revolution
It used to be thought that cancer was simply due to too many cells being created. Professor David Vaux discovered that some cancers are due to the opposite process: not the birth of too many cells, but to genetic errors that prevent unwanted cells from dying when they should.
In 1987, when Vaux began his PhD in the laboratory of Professor Jerry Adams and Suzanne Cory, “it was generally thought that cancer genes were just normal genes that regulated cell growth. But when they were mutated, they caused cells to proliferate in an uncontrolled way.”
His task was to work on a gene called Bcl-2, which was suspected to be a cancer gene because it was often altered in lymphomas, a type of malignancy of the white blood cells. He wanted to prove whether it was a cancer gene, and to find out how it actually worked. “The expectation was that this gene encoded a growth regulator that caused cancer when it was mutated,” he says.
“My task was to find out, ‘Is Bcl-2 another oncogene [cancer gene]? If you put it into cell and turn it on, does it make the cell divide more rapidly and generate leukaemia cells?’”
Disappointment leads to amazing find
The answer, initially, was a disappointing No. But, after persisting with different kinds of testing, Vaux discovered how Bcl-2 worked: “Rather than stimulating cell division, it stopped cells from being able to kill themselves.
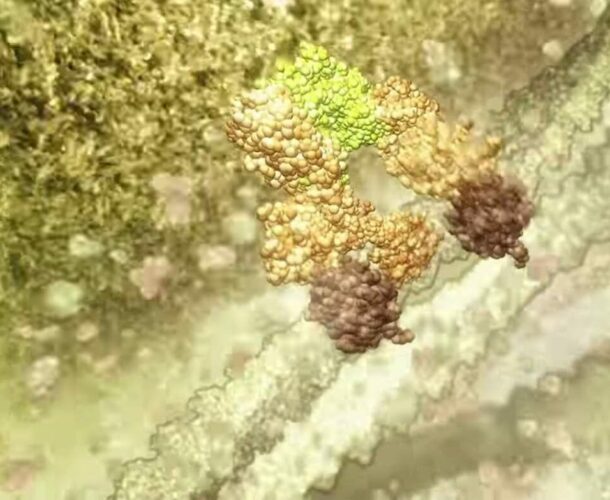
“In our bodies we produce a million new cells every second, in the skin, intestines, blood etc. But, so you don’t end up the size of a planet, you also need to get rid of a million cells a second. Normal cells activate an inbuilt self-destruct mechanism called apoptosis. It’s sort of a time-bomb that allows a cell to kill itself. We discovered that failure of cell death was an important cause of cancer in humans.”
Vaux made transgenic mice that expressed lots of Bcl-2, thinking, “These mice will develop lymphoma.” It turned out that while the mice did have too many B cells (the type of white blood cells that make antibodies), they usually didn’t die of lymphoma. His colleague, Professor Andreas Strasser, found that these mice died of a disease that resembled lupus (where the body’s immune system becomes overactive and attacks healthy tissue). This suggested that cell death is needed to remove cells that can cause autoimmune disease.
A milestone in cancer
Vaux’s 1988 paper showing that Bcl-2 is an inhibitor of apoptosis sparked huge interest in the field of cell death and was hailed as a milestone in the study of cancer genes. In 2003 he was awarded the Victoria Prize for his work.
Apoptosis has since become a major focus of research, both at the Walter and Eliza Hall Institute and around the world, with relevance not just to cancer and autoimmune diseases but, possibly, also to heart attacks and stroke. “The fact that cells kill themselves had been recognised for 100 years, but it was something hardly anyone was studying,” he says. “Now, 25,000 papers a year are written about it.”
Vaux, now assistant director of the Walter and Eliza Hall Institute, is still investigating cell death. A collaboration between the institute and biotech companies Abbvie and Genentech, a member of the Roche group, has led to a new anti-cancer agent. Called BH3-mimetics, they bind to Bcl-2 and stop it from working, so that the cancer cells’ self-destruct process can go ahead.
Vaux says, “The icing on the cake would be if these drugs actually work and if, after 27 years of work, some of this research could be of practical use.”